Learning Objectives
- Describe the structure and function neurons
- Explain the role of membrane potential in neuron communication
- Identify and differentiate between the different types of ion channels that regulate neuron function
- Interpret an action potential graph and explain the behavior of ion channels underlying each step of the action potential
- Describe the structure and function of neuronal synapses, the process that leads to neurotransmitter release, and the role of neurotransmitters at the synapse
- Differentiate between excitatory and inhibitory post-synaptic potentials (EPSPs and IPSPs), and predict the results of multiple co-occurring PSPs
Neurons Send and Receive both Chemical and Electrical Signals
The information below was adapted from OpenStax Biology 35.1 and Khan Academy AP Biology The neuron and nervous system. All Khan Academy content is available for free at www.khanacademy.org
Neurons are specialized cells that can receive and transmit chemical or electrical signals, and they are supported by cells called glia, which provide support functions for the neurons.
Neurons communicate via both electrical signals and chemical signals:
- The electrical signals are action potentials, which transmit the information from one of a neuron to the other. An action potential is a rapid, temporary change in membrane potential (electrical charge), and it is caused by sodium rushing to a neuron and potassium rushing out.
- The chemical signals are neurotransmitters, which transmit the information from one neuron to the next. Neurotransmitters are chemical messengers which are released from one neuron as a result of an action potential; they cause a rapid, temporary change in the membrane potential of the adjacent neuron to initiate an action potential in that neuron.
Neuron Structure
The structure of a neuron supports its function in sending and receiving both chemical and electrical messages:
- Soma (cell body): Each neuron has a cell body that contains its nucleus and other cellular organelles and components.
- Dendrites: Dendrites are the structures where neurons receive signals from other neurons (in some cases, neurons may also receive signals directly at the soma). Dendrites are tree-like structures that extend away from the cell body to receive neurotransmitters from other neurons. Some types of neurons do not have any dendrites, some types of neurons have multiple dendrites. Dendrites can have small protrusions called dendritic spines, which further increase surface area for possible connections with other neurons.
- Axon: The axon is the structure where a neuron transmits an action potential to ultimately reach the next neuron. The axon is a tube-like structure that propagates the integrated signal to specialized endings called axon terminals. Neurons usually have one or two axons.
- Some axons are covered with myelin, which acts as an insulator to minimize dissipation of the electrical signal as it travels down the axon; myelination greatly increases the speed of conduction. This insulation is important as the axon from a human motor neuron can be as long as a meter, from the base of the spine to the toes. The myelin sheath is not actually part of the neuron; it is produced by a specialized type of cell called a glial cell, which functions to support neurons. Along myelinated axons there are periodic gaps in the myelin sheath called nodes of Ranvier, which are sites where the signal is “re-charged” as it travels along the axon.
- Synapses: A synapse is the name of the location where two neurons are almost in contact with each other, and where a signal is transmitted from one neuron to the next. On one side of a synapse is the axon terminal from the pre-synaptic neuron (the neuron that is sending the signal); on the other side is a dendrite or dendritic spine of the post-synaptic neuron (the neuron that is receiving the signal). In between is the synaptic cleft, a small gap between the two synapsed neurons where neurotransmitters are released from one neuron to pass the signal to the next neuron.
- Axon hillock: The axon hillock is the site where an action potential initiates within a neuron; it is also the site that effectively controls whether or not an action potential will occur. A neuron doesn’t necessarily initiate an action potential every time it receives a a signal at a synapse. In fact, each neuron can share synapses with thousands to hundreds of thousands of other neurons, and they may be sending conflicting signals at the same time. These conflicting signals are “integrated” at a location called axon hillock, which is located between the neuron cell body and the start of the axon. Integration at the axon hillock determines whether the neuron will initiate an action potential (more on this later in this reading).
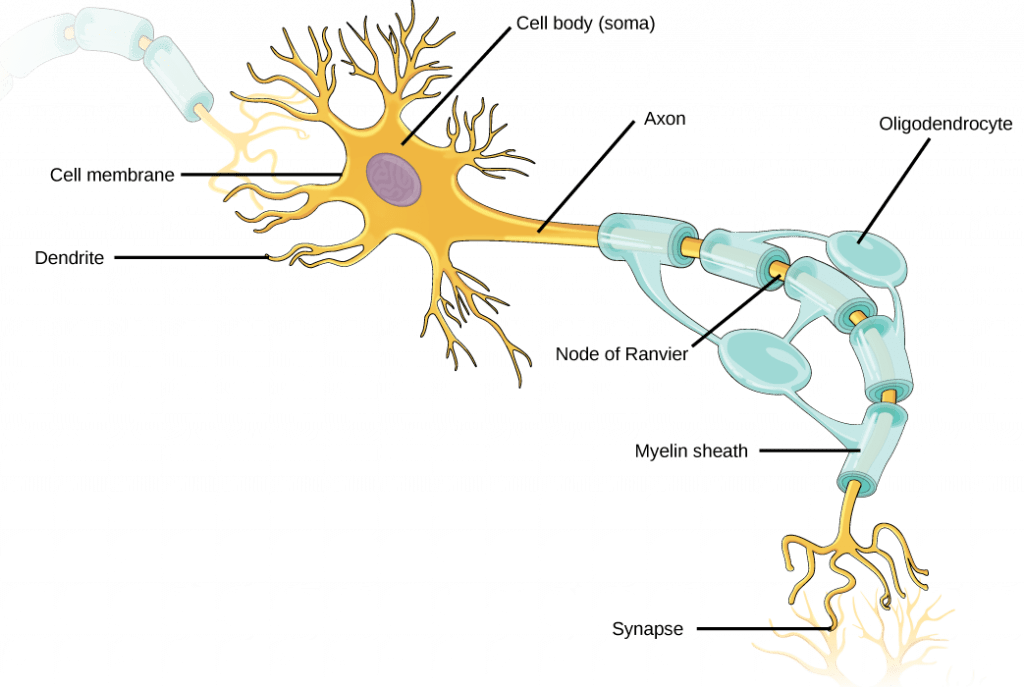
It is important to note that a single neuron does not act alone: neuronal communication depends on the connections that neurons make with one another (as well as with other cells, like muscle cells). Dendrites from a single neuron may receive synaptic contact from many other neurons. For example, dendrites from a neurons in the cerebellum of the brain are thought to receive contact from as many as 200,000 other neurons.
Membrane Potential and Neuron Communication
The information below was adapted from OpenStax Biology 35.2 and OpenStax Anatomy & Physiology 3.1
Transmission of a signal between neurons is generally carried by a chemical called a neurotransmitter. But transmission of a signal within a neuron (from dendrite to axon terminal) is carried by a brief change in membrane potential, or membrane electrical charge, called an action potential. This communication is possible because each neuron has a charged cellular membrane (a voltage difference between the inside and the outside), and the charge of this membrane can change in response to neurotransmitter molecules released from other neurons and environmental stimuli. The resting membrane potential and action potentials are controlled by the different types of ion channels. Below we’ll discuss these two inter-related phenomena together:
- Resting potential: the membrane potential (electrical charge) in a neuron that is not currently transmitting a signal; maintained by the sodium potassium pump and potassium leak channels
- Action potential: a brief depolarization (reduction in magnitude of the charge) along the neuron’s axon; action potentials are all-or-nothing (they do not have degrees of magnitude); regulated by voltage-gated sodium channels, voltage-gated potassium channels, and the sodium potassium pump
- Neurotransmitters: the chemical messengers that communicate between adjacent neurons; release of neurotransmitters from one neuron will either help depolarize or hyperpolarize (increase the magnitude of the charge) the adjacent neuron, making an action potential either more or less likely to occur in the next neuron
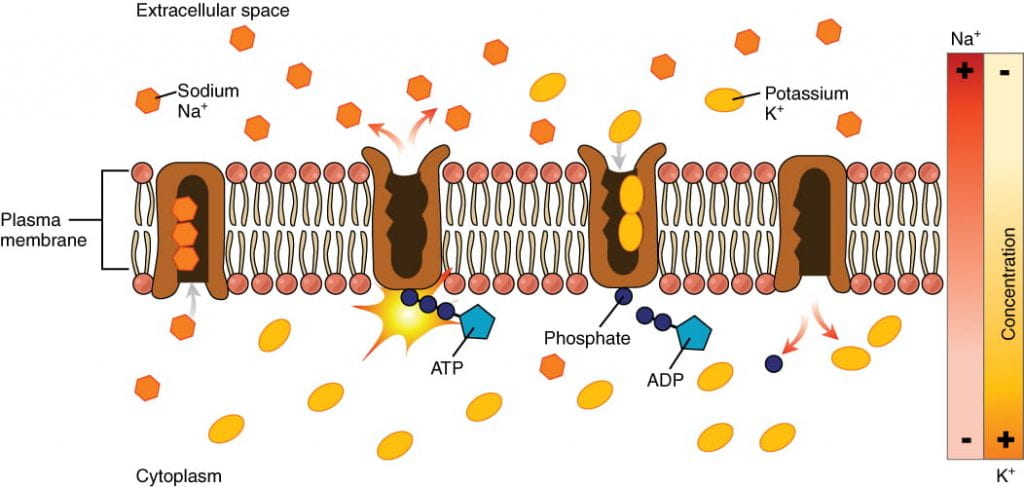
The Resting Potential: Sodium-Potassium Pumps and Potassium Leak Channels
Small charged molecules and ions are unable to cross a cell’s lipid bilayer membrane on their own; ions must pass through special proteins called ion channels that span the membrane and regulate the relative concentrations of different ions inside and outside the cell. Cells can use energy to preferentially move certain ions either inside or outside of the membrane, setting up a difference in ion charge across the membrane, where one side is relatively more negative and the other side is relatively more positive. The difference in total charge between the inside and outside of the cell is called the membrane potential.
The membrane potential of a neuron at rest is negatively charged: the inside of a cell is approximately 70 millivolts more negative than the outside (-70 mV, note that this number varies by neuron type and by species). This voltage is called the resting membrane potential; it is caused by differences in the concentrations of ions inside and outside the cell. The resting potential is established and maintained by two main processes: an ATP-powered ion channel called the sodium-potassium pump, and a passive ion channel called the potassium leak channel:
- The sodium-potassium pump: which is also called Na+/K+ ATPase, transports sodium out of a cell while moving potassium into the cell. The Na+/K+ pump is an important ion pump found in the membranes of many types of cells. These pumps are particularly abundant in nerve cells, which are constantly pumping out sodium ions and pulling in potassium ions to maintain an electrical gradient across their cell membranes. An electrical gradient is a difference in electrical charge across a space. In the case of nerve cells, for example, the electrical gradient exists between the inside and outside of the cell, with the inside being negatively-charged (at around -70 mV) relative to the outside. The negative electrical gradient is maintained because each Na+/K+ pump moves three Na+ ions out of the cell and two K+ ions into the cell for each ATP molecule that is used. This process is so important for nerve cells that it accounts for the majority of their ATP usage.
- The potassium leak channels allow the potassium to diffuse down its concentration gradient and leak out of the cell. (Neurons also have sodium leak channels, but they have far more potassium leak channels than sodium leak channels, so will focus on potassium leak channels for the purposes of this course).As a result of the positively charged potassium ions leaving the cell, the interior of the cell is negatively charged relative to the outside of the cell.
The combined effects of the sodium-potassium pump and the potassium leak channels is that the interior of the cell is more negative than the outside of the cell. It should also be noted that chloride ions (Cl-) tend to accumulate outside of the cell because they are repelled by negatively-charged proteins within the cytoplasm.
| Ion Concentration Inside and Outside Neurons | |||
|---|---|---|---|
| Ion | Extracellular concentration (mM) | Intracellular concentration (mM) | Ratio outside/inside |
| Na+ | 145 | 12 | 12 |
| K+ | 4 | 155 | 0.026 |
| Cl- | 120 | 4 | 30 |
| Organic anions (A-) | — | 100 |
This video describes the role of the sodium/potassium pump and potassium leak channels in establishing and maintaining the membrane resting potential:
The Action Potential: Voltage-Gated Sodium Channels, Voltage-Gated Potassium Channels, and the Sodium Potassium Pump
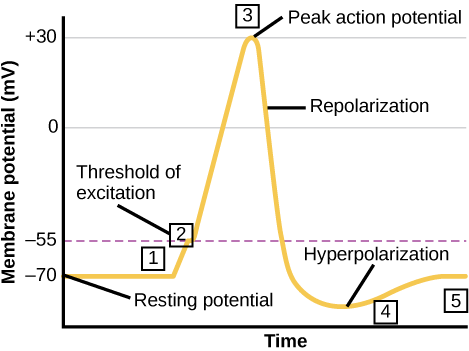
When we talk about neurons “firing” or being “active,” we’re talking about the action potential: a brief, positive change in the membrane potential along a neuron’s axon. When an action potential occurs, the neuron sends the signal to the next neuron in the communication chain, and, if an action potential also occurs in the next neuron, then the signal will continue being transmitted. What causes an action potential? When a neuron receives a signal from another neuron (in the form of neurotransmitters, for most neurons), the signal causes a change in the membrane potential on the receiving neuron. The signal causes opening or closing of voltage-gated ion channels, channels that open or close in response to changes in the membrane voltage. The opening of voltage-gated ion channels causes the membrane to undergo either a hyperpolarization, where the membrane potential increases in magnitude (becomes more negative) or a depolarization, where the membrane potential decreases in magnitude (becomes more positive). Whether the membrane undergoes a hyperpolarization or a depolarization depends on the type of voltage-gated ion channel that opened.
It is important to note that not all depolarizations result in an action potential. The signal must cause a depolarization that is large enough in magnitude to overcome the threshold potential, or the specific voltage that the membrane must reach for an an action potential to occur. The threshold potential is usually about -55 mV, compared to the resting potential of about -70 mV. If the threshold potential is reached, then an action potential is initiated at the axon hillock in the following stages, through the action of the associated ion channels:
- Depolarization and voltage-gated sodium channels: voltage-gated sodium channels open quickly after depolarization past the threshold potential. The phrase “voltage-gated” refers to the idea that these channels only open if the membrane reaches a specific voltage; they are “gated” by the voltage. Depolarization of the axon hillock to -55 mV is sufficient to open the “gate” of the voltage-gated sodium channels. As sodium rushes into the axon (influx), the inside becomes relatively electrically positive (approximately +30 mV, compared to the initial resting potential of apprximately -70 mV).
- Repolarization and voltage-gated potassium channels: shortly after the initial depolarization, the voltage-gated sodium channels close and remain closed for about 1-2 milliseconds. During this period, the voltage-gated sodium channels are “refractory” to opening, meaning they cannot open even if the membrane were at the appropriate voltage to allow them to. Voltage-gated potassium channels then open, allowing potassium to rush out of the axon (efflux) and causing the membrane to repolarize (become more negative). Just like the voltage-gated sodium channels that initiated the action potential, the voltage-gated potassium channels can only open when the membrane reaches a specific voltage.
- Hyperpolarizaton and voltage-gated potassium channels: potassium continues leaving the axon to the point that the membrane potential dips below the normal resting potential. Sodium channels return to their resting state, meaning they are ready to open again if the membrane potential again exceeds the threshold potential.
- Reset resting potential and the sodium potassium pump: The sodium-potassium pump and potassium leak channels reset the locations of sodium and potassium ions, reestablishing the membrane potential to allow another action potential to fire. (These ion channels never stopped working during the action potential, but their effects were overwhelmed by the activities of the voltage-gated sodium and voltage-gated potassium ions.)
These steps are illustrated here:
The video below provides a discussion of voltage-gated ion channels
And here is a more in-depth discussion of the action potential:
And an overview of action potential propagation:
There are a few important universal features of action potentials:
- The action potential travels down the axon, proceeding as a wave of depolarization. The image above shows a trace of an action potential at a single point in the membrane of an axon; the same pattern repeats down the entire length of the axon until it reaches the synapse, shown in te
- Action potentials always proceed in one direction only, from the cell body (soma) to the synapse(s) at the end of the axon. Action potentials never go backward, due to the refractory period of the voltage-gated ion channels, where the channels cannot re-open for a period of 1-2 milliseconds after they have closed. The refractory period forces the action potential to travel only in one direction.
- Action potentials do not vary in magnitude or speed; they are “all-or-nothing.” When a given neuron fires, the action potential always depolarizes to the same magnitude and always travels at the same speed along the axon. There is no such thing as a bigger or faster action potential. The parameter that can vary is the frequency of action potentials, or how many action potentials occur in a given amount of time.
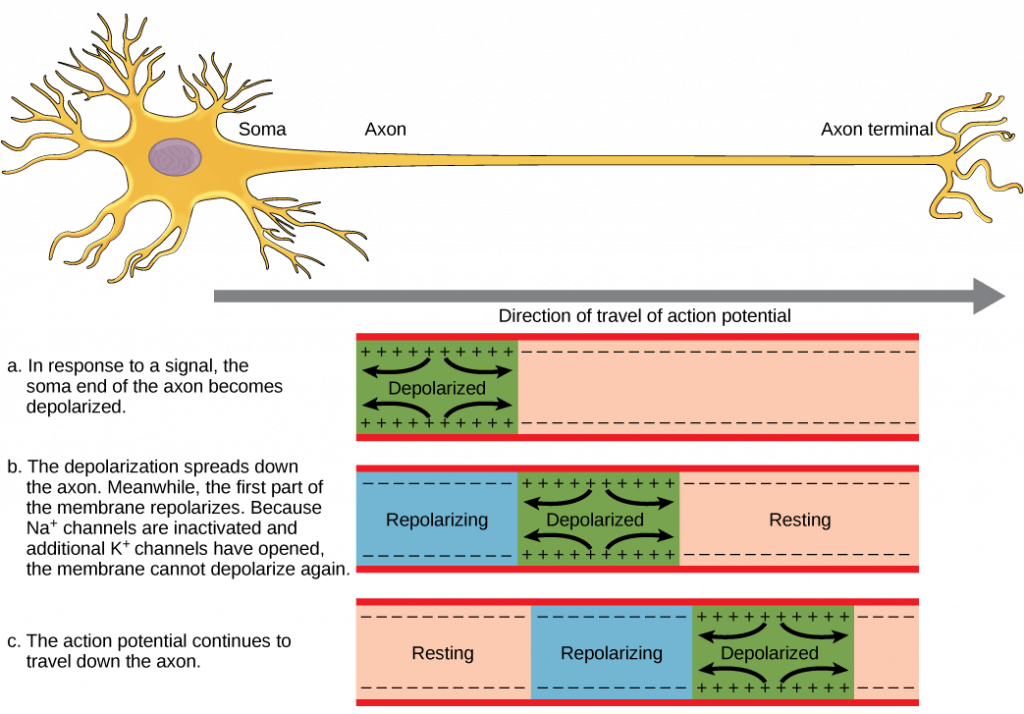
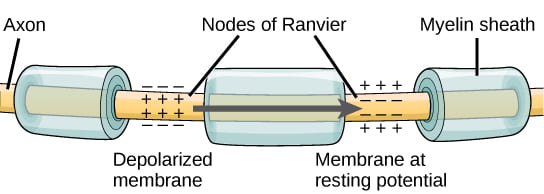
As noted above, the magnitude or speed of the action potential for a given neuron never varies; however, some neurons have faster action potentials than others. In invertebrates, this difference is often due to axon diameter, where larger axons have faster conduction of action potentials. In vertebrates, this difference is typically due to myelination of the neuron’s axon. Myelin acts as an insulator that prevents current from leaving the axon; this increases the speed of action potential conduction. The nodes of Ranvier, illustrated below, are gaps in the myelin sheath along the axon. These unmyelinated spaces are about one micrometer long and contain voltage gated Na+ and K+ channels. Flow of ions through these channels, particularly the Na+ channels, regenerates the action potential over and over again along the axon. This “jumping” of the action potential from one node to the next is called saltatory conduction. Nodes of Ranvier also save energy for the neuron since the channels only need to be present at the nodes and not along the entire axon.
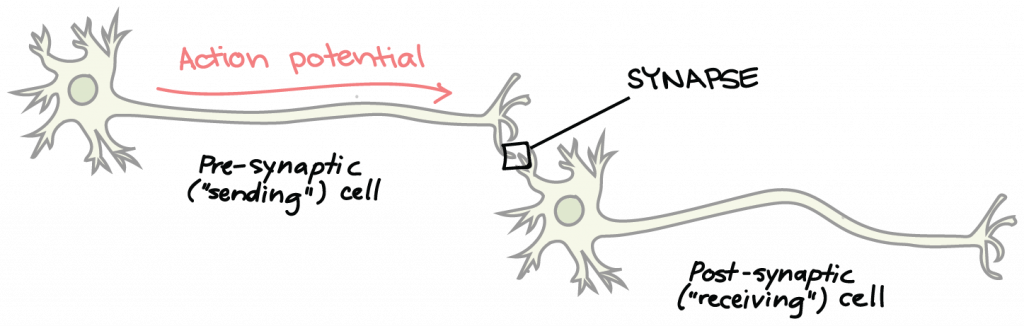
The Synapse: Voltage-Gated Calcium Channels, Synaptic Vesicles, and Neurotransmitters
Neurons are not in direct physical contact with each other, but instead come into very close proximity at a structure called the synapse. The neuron sending a signal to the next is called the presynaptic neuron, and the neuron receiving a signal is called the postsynaptic neuron, shown here:
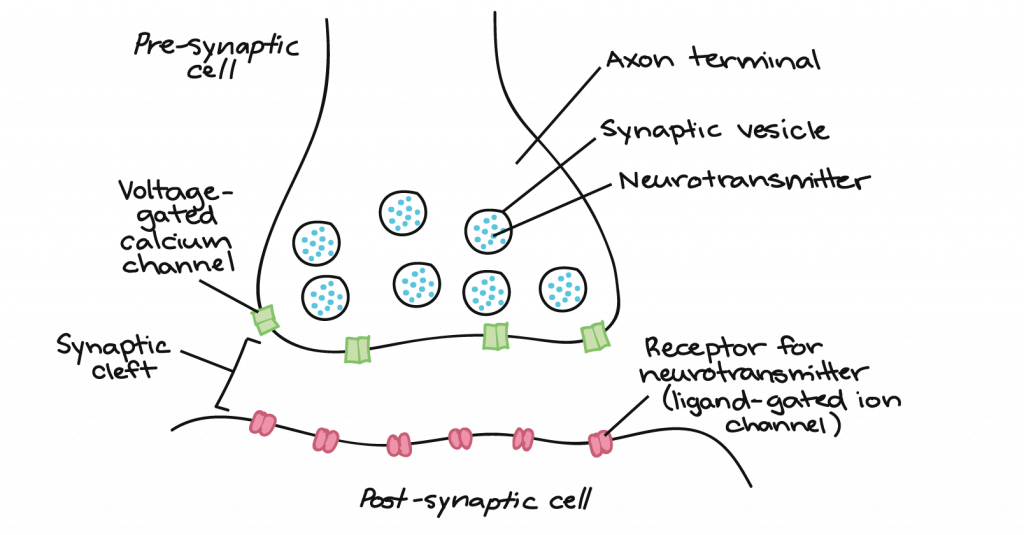
There is a small gap between the two neurons called the synaptic cleft, where neurotransmitters are released by the presynaptic neuron to transmit the signal to the postsynaptic neuron, shown here:
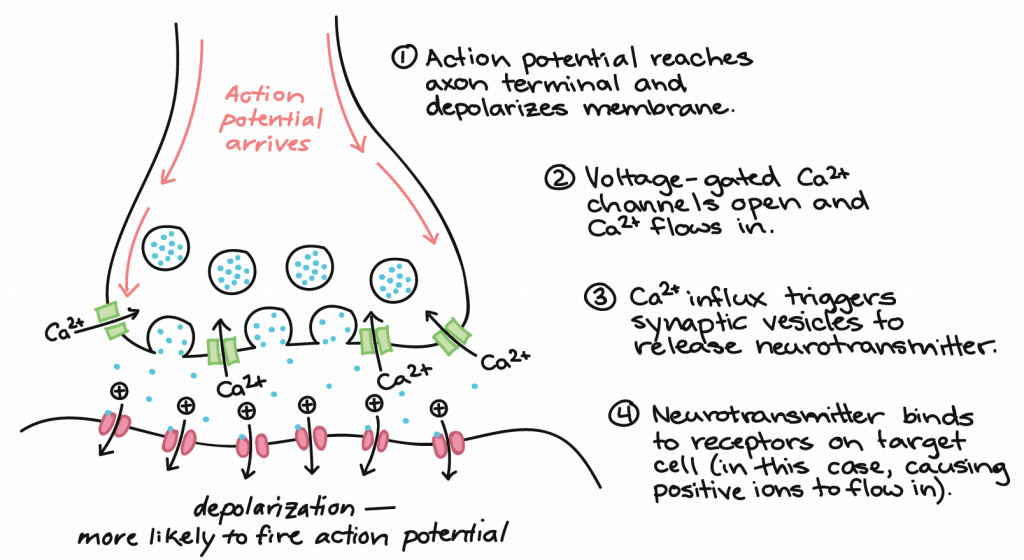
How does synaptic transmission work? Once the action potential reaches the end of the axon, it propagates into the pre-synaptic terminal where the following events occur in sequence:
- The action potential depolarizes the membrane and opens voltage-gated Na+ channels. Na+ ions enter the cell, further depolarizing the presynaptic membrane.
- This depolarization causes voltage-gated calcium channels to open in the presynaptic neuron, allowing calcium ions to enter the presynaptic neuron at the synpase.
- Calcium ions entering the presynaptic neuron cell initiate a signaling cascade that causes small membrane-bound vesicles, called synaptic vesicles, to fuse with the presynaptic membrane. The synaptic vesicles contain neurotransmitter molecules.
- Fusion of a vesicle with the presynaptic membrane causes neurotransmitter to be released into the synaptic cleft, the extracellular space between the presynaptic and postsynaptic membranes. The neurotransmitter diffuses across the synaptic cleft and binds to receptor proteins on the postsynaptic membrane.
This process is illustrated below:
Once neurotransmission has occurred, the neurotransmitter must be removed from the synaptic cleft so the postsynaptic membrane can “reset” and be ready to receive another signal. This can be accomplished in three ways:
- the neurotransmitter can diffuse away from the synaptic cleft
- the neurotransmitter can be degraded by enzymes in the synaptic cleft
- the neurotransmitter can be recycled (sometimes called reuptake) by the presynaptic neuron.
This video walks through the process of signal communication across a chemical synapse:
Post-Synaptic Potentials
What happens to the post-synaptic neuron when neurotransmitters released by the pre-synaptic neuron bind to receptors on the post-synaptic neuron? It depends:
- Excitatory postsynaptic potentials (EPSPs) depolarize the post-synaptic membrane, making the postsynaptic neuron more likely to fire an action potential. For example, when acetylcholine is released at the synapse between a nerve and muscle (called the neuromuscular junction) by a presynaptic neuron, it causes postsynaptic Na+ channels to open. Na+ enters the postsynaptic cell and causes the postsynaptic membrane to depolarize.
- Inhibitory postsynaptic potentials (IPSPs) hyperpolarize the post-synaptic membrane, making the postsynaptic neuron less likely to fire an action potential. For example, when the neurotransmitter GABA (gamma-aminobutyric acid) is released from a presynaptic neuron, it binds to and opens Cl– (chloride) channels. Cl– ions enter the cell and hyperpolarizes the membrane.
While action potentials are “all-or-nothing,” as noted above, EPSPs and IPSPs are graded; they vary in magnitude of depolarization or hyperpolarization, as illustrated below:
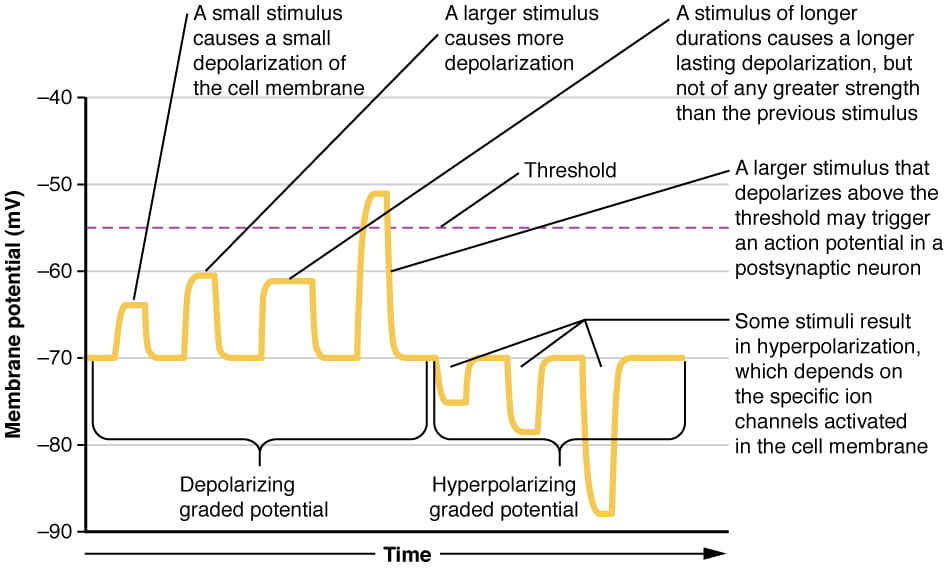
A single EPSP typically isn’t strong enough to induce an action potential in the postsynaptic neuron on its own, and multiple presynaptic inputs must create EPSPs around the same time for the postsynaptic neuron to be sufficiently depolarized to fire an action potential. This process is called summation or integration and occurs at the axon hillock, as illustrated below. In addition, each neuron often has inputs from many presynaptic neuron – some excitatory and some inhibitory – so IPSPs can cancel out EPSPs and vice versa. It is the net change in postsynaptic membrane voltage that determines whether the postsynaptic cell has reached its threshold of excitation needed to fire an action potential. Together, synaptic summation and the threshold for excitation at the axon hillock act as a filter so that random “noise” in the system is not transmitted as important information.
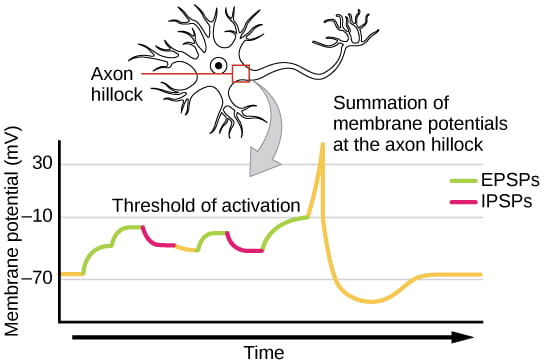
The video below provides an over view of summation over time and space:
Here are two final videos to help you put this all together (and in a more engaging way than any of the videos above). Note that these videos do not provide any new information, but they may help you better integrate all the information previously discussed:

