Learning Objectives
- Identify the hormones that regulate specific plant behaviors and describe their role in that behavior, including auxin, cytokinin, gibberellin, abscisic acid, and ethylene
- Recognize the stimulus (blue light, red light, far-red light, gravity, water, water stress, touch) that provokes a specific plant behavior, including phototropism, gravitropism, germination, stomatal closing, and thigmotropism
- Describe the pathways that regulate specific plant behaviors, including phototropism, gravitropism, germination, and water stress
- Interpret and predict outcomes of experiments manipulating plant signaling pathways
Hormones in plants
The information below was adapted from OpenStax Biology 30.6
A plant’s sensory response to external stimuli relies on chemical messengers (hormones). Plant hormones affect all aspects of plant life, from flowering to fruit setting and maturation, and from phototropism to leaf fall. Just as in animals, hormones are signaling molecules which are present in very small amounts, transported throughout the plant body, and only elicit in responses in cells which have the appropriate hormone receptors. In plants, hormones travel large throughout the body via the vascular tissue (xylem and phloem) and cell-to-cell via plasmodesmata.
Potentially every cell in a plant can produce plant hormones. In contrast, many animal hormones are produced only in specific glands. Plants do not have specialized hormone-producing glands.
Hormones regulate a variety of plant behaviors in response to different stimuli or environmental conditions. This page is divided into two parts:
- Part 1 describes some of the hormones that initiate and regulate plant behaviors
- Part 2 describes the stimuli that provoke these responses and the pathways that regulate the responses.
Throughout this reading, you should aim to recognize both the stimuli that provoke a specific behavior, as well as the hormones and (when described) the signaling pathway that mediates the response.
Part 1: (A Small Subset of) Hormones Regulating Plant Responses
In this section, we’ll describe one plant hormone at a time and briefly describe all the plant behaviors associated with that hormone. In the section following, we’ll then describe particular stimulus that initiates a plant behavior and the pathway that regulates that response. Please note, there are many (many) other plant hormones which are beyond the scope of this course and are not discussed in this reading.
- Auxin: the ‘youth’ hormone and the master growth regulator
- Tissues with high levels of auxins tend to behave as ‘younger’ tissues: they are areas of new growth and they don’t exhibit signs of aging (senescence).
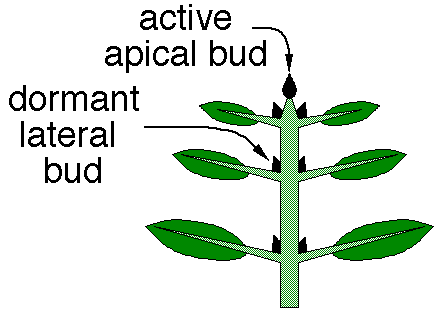
- Auxins are the main hormones responsible for cell elongation in phototropism (movement in response to light) and gravitropism (movement in response to gravity), apical dominance (inhibition of lateral bud formation), and inhibition of abscission (leaf falling) are other plant responses under the direct or indirect control of auxins.
- Synthetic auxin is used as a rooting hormone to promote growth of roots on cuttings and detached leaves.
- Cytokinin: the ‘cell division’ hormone
- Cytokinin promotes cytokinesis (cell division). Cytokinins are most abundant in growing tissues, such as roots, embryos, and fruits, where cell division is occurring.
- The effects of cytokinins are often also influence by auxin; for example, apical dominance seems to result from a balance between auxins that inhibit lateral buds, and cytokinins that promote bushier growth.
- Gibberellin: the ‘growth’ hormone; stem, fruit, and seed growth
- Gibberellins (GAs) stimulate shoot elongation, seed germination, and fruit and flower maturation; GAs are synthesized in the root and stem apical meristems, young leaves, and seed embryos.
- GAs also break dormancy (a state of inhibited growth and development) in the seeds of plants that require exposure to cold or light to germinate.
- Maturing grapes are routinely treated with GA to promote larger fruit size.
- Abscisic Acid (ABA): the ‘dormancy‘ hormone
- ABA accumulates as a response to stressful environmental conditions, such as dehydration, cold temperatures, or shortened day lengths. Its activity counteracts many of the growth-promoting effects of GAs and auxins.
- ABA causes the abscission (dropping) of leaves, inhibits stem elongation, induces dormancy in lateral buds and seeds, and closes stomata in short-term drought conditions.
- Ethylene: the ‘aging’ (senescence) hormone; leaf abscission, fruit ripening
- Ethylene is unusual as a hormone because it is a volatile gas (C2H4).
- Ethylene promotes fruit ripening, flower wilting, and leaf abscission (falling)
- Aging tissues (especially older leaves) and nodes of stems produce ethylene,
- Ethylene is widely used in agriculture. Commercial fruit growers control the timing of fruit ripening with application of the gas. Horticulturalists inhibit leaf dropping in ornamental plants by removing ethylene from greenhouses using fans and ventilation.
This video describes the activities of both gibberellins and abscisic acid (watch from 11:30 to 16:00):
This video provides a quick summary of the different roles of ethylene in plants:
Part 2: Plant Responses to Stimuli
In the section above, we’ve listed a set of plant hormones and briefly identified the behaviors they regulate. In the section below, we’ll describe the different stimuli that plants can respond to, the responses to these stimuli, and the hormones that play a role in the response pathway. In some cases, we will also go into some depth describing the pathways that regulate these responses. In other words, the section below explains how these hormones regulate the behaviors described in the previous section.
Plant Responses to Light: Phototropism and Germination
Plants are generally capable of detecting and responding to at least three wavelengths of light: blue light, red light, and far-red light. The different wavelengths are detected by different photoreceptors, which are comprised of a protein covalently bonded to a light-absorbing pigment called a chromophore. Together, the two are called a chromoprotein. The behaviors regulated by light stimuli include:
- phototropism (movement toward light)
- stem elongation (growth)
- germination (seed sprouting)
- photoperiodism (flowering in response to length of day)
We’ll discuss these in turn below:
Response to Blue Light: Phototropism
Phototropism is movement toward or away from light. Tropism means movement, and photo means light, so “phototropism” is “movement in response to light.”
Phototropins are the chromoproteins (proteins containing pigmented co-factors) which are responsible for mediating the phototropic response. Phototropins also control opening of stomata to permit gas exchange (and thus photosynthesis).
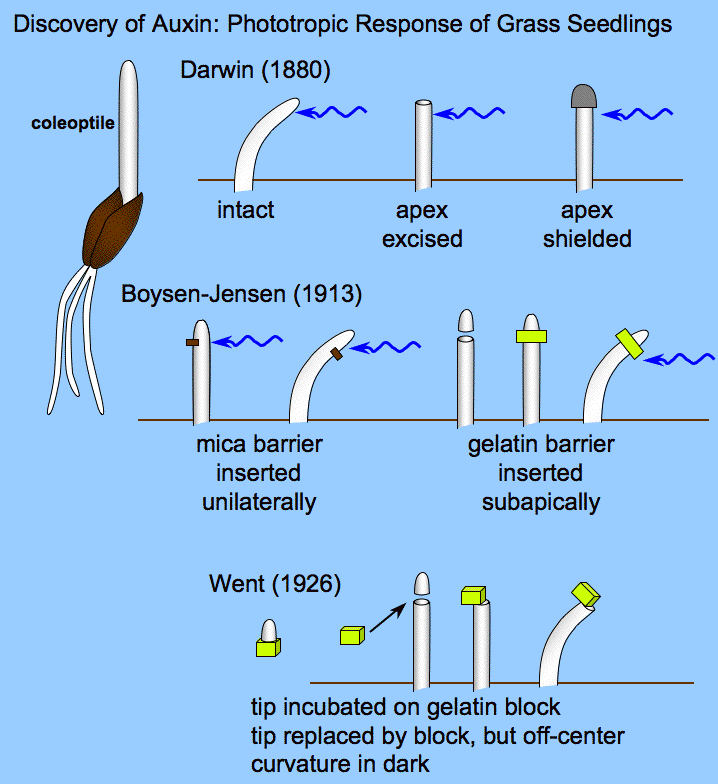
Charles Darwin and his son Francis determined that light is detected by the tip of the plant (the apical meristem), but that the response (bending) takes place in a different part of the plant. They concluded that the signal must have to travel from the apical meristem to the base of the plant to cause the bending. We now know that the detection of light in the apical meristem occurs via phototropins called phot1 and phot2, which specifically detect blue light.
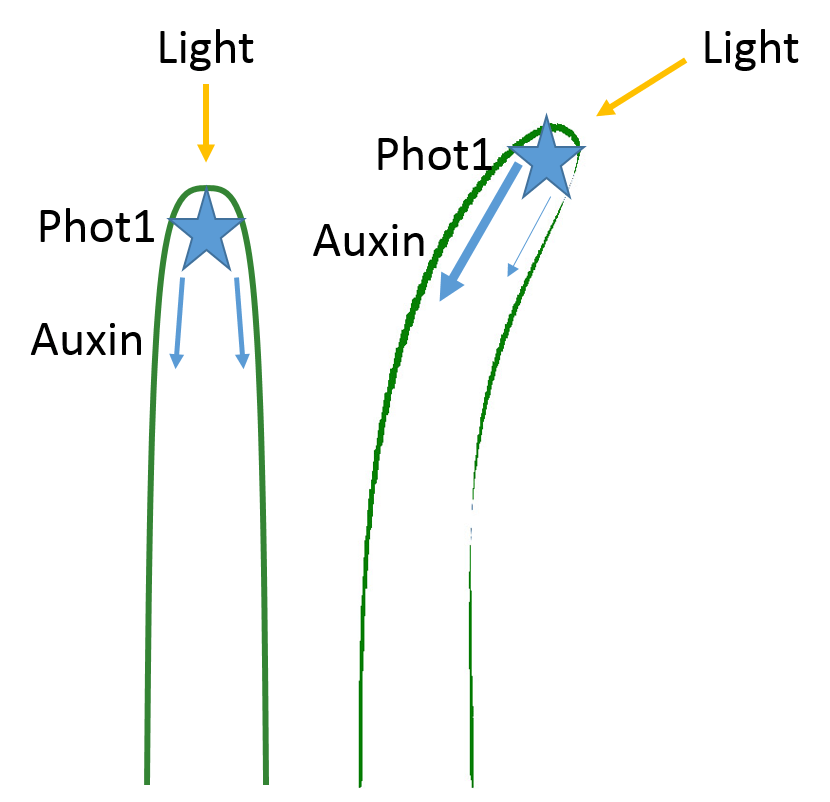
In 1913, Peter Boysen-Jensen cut off the tip of a seedling, covered the cut section with a layer of gelatin (essentially Jell-O), and then replaced the tip. The cut seedling could still bend toward the light. But when he inserted an impermeable barrier (instead of Jell-O) between the tip and the cut base, the seedling could no longer bend in response to light. Later experiments showed that the signal traveled on the shaded side of the seedling: when the barrier was inserted only on the illuminated side, the plant could still bend towards the light. Therefore, the chemical signal was a growth stimulant because the phototropic response involved great cell elongation on the shaded side than on the illuminated side, which resulted in the stem bending toward the light. We now know that the chemical signal is the plant hormone auxin. (Auxin also controls many other plant behaviors not described here.)
Auxin stimulates cell elongation on the shady side of the stem through a process called the acid growth hypothesis: Auxin causes cells to activate proton pumps, which then pump protons out of the cells and into the space between the plasma membrane and the cell wall. The movement of protons into the extracellular space does two things:
- The lower pH activates expansin, which breaks the links between the cellulose fibers in the cell walls, making them more flexible.
- The high concentration of protons causes sugars to move into the cell, which then creates an osmotic gradient where water moves into cell causing the cell to expand.
To sum up, the phototropic response works like this:
- the phototropins phot1 and phot2 are present in the plant apical meristem
- when activated by blue light, phot1 and phot2 cause accumulation of auxin on the shaded side of the plant.
- auxin promotes cell elongation due to weakening of the cell wall combined with influx of water (which literally stretches the cells)
- because the cell expansion occurs only on the shaded side of the stem, the plant bends away from the shade and toward the light
This video provides a concise summary of auxin’s role in phototropism and the acid growth hypothesis (note that the video ends early to direct you to another study site, but the portion available here covers what you need to understand for this course):
Response to Red Light: Growth or Germination
Blue light promotes stem bending, but red light (as opposed to far-red light) promotes stem elongation, or growth. Why? Red light indicates full sun to a plant, while far-red light indicates that a plant is being shaded out by another plant. This is because unfiltered, full sunlight contains much more red light than far-red light. In other words, plants use the red vs far-red light detection to grow away from shade and towards light.
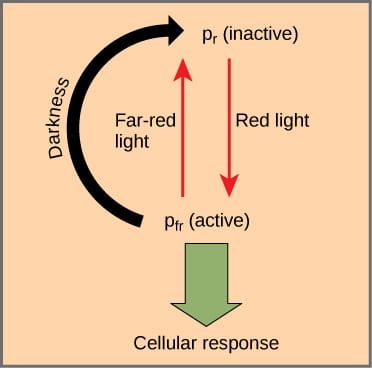
The chromoprotein responsible for red/far-red light detection is called phytochrome. Phytochrome acts like a reversible switch that can absorb either red light or far-red light. Photochrome has two photo-interconvertible forms, which are named for the wavelength of light they are capable of absorbing; NOT the wavelength of light that they just asbsorbed:
- Pr (phytochrome red) is capable of absorbing red light (~667 nm)
- Pfr (phytochrome far-red) is capable of absorbing far-red light (~730 nm).
- When the Pr form absorbs red light, it is immediately converted to Pfr; and when Pfr absorbs far-red light, it is quickly converted back to Pr.
- Pfr is the physiologically active form of the protein. Because phytochrome is in the Pfr state after exposure to red light, this means that exposure to red light turns the phytochrome “on.”
- Exposure to far-red light inhibits phytochrome activity.
The behaviors that the phytochrome system regulates include plant growth and seed germination (and many others not discussed here):
- Phytochrome stimulates plant growth toward red light in combination with the hormones cytokinin, which promotes cell division, and gibberellin, which promotes stem elongation. (Interestingly, cytokinin is only capable of promoting cell division when it is also in the presence of auxin, which is present at apical meristems but not other locations in the plant. Auxin also regulates levels of gibberellin.)
- The phytochrome system also regulates seed germination in many plant species (illustrated below). As we’ve previously discussed, the seeds of many plants go into a dormant state after fertilization, in part to ensure that the seed germinates at a time and in a place that the seedling is more likely to successfully survive. There are many different signals that can trigger seed germination, depending on the plant species. For many plant species, this signal is red light, as red light provides a signal that the seed is in a good location for access to sunlight after germination. In contrast, a seed that germinates in shaded areas, or too deep under the soil to reach the sunlight, is likely to die soon after germination. In the dark, phytochrome is in the Pr (inactive form) and the seed will not germinate; it will only germinate if exposed to light at the surface of the soil. Upon exposure to light, Pr is converted to Pfr, Pfr signaling causes transcription of the gene that encodes amylase, an enzyme that breaks down starches stored in the seed into simple sugars, and then germination proceeds. (Note that some species of plants initiate germination through an light-independent process regulated by the hormone gibberellin, which is further described below.)

Response to Gravity: Gravitropism
Whether or not they germinate in the light or in total darkness, shoots usually sprout up from the ground, and roots grow down into the ground. A plant laid on its side in the dark will send shoots upward when given enough time. Gravitropism ensures that roots grow into the soil and that shoots grow toward sunlight. Growth of the shoot apical tip upward is called negative gravitropism, whereas growth of the roots downward is called positive gravitropism.
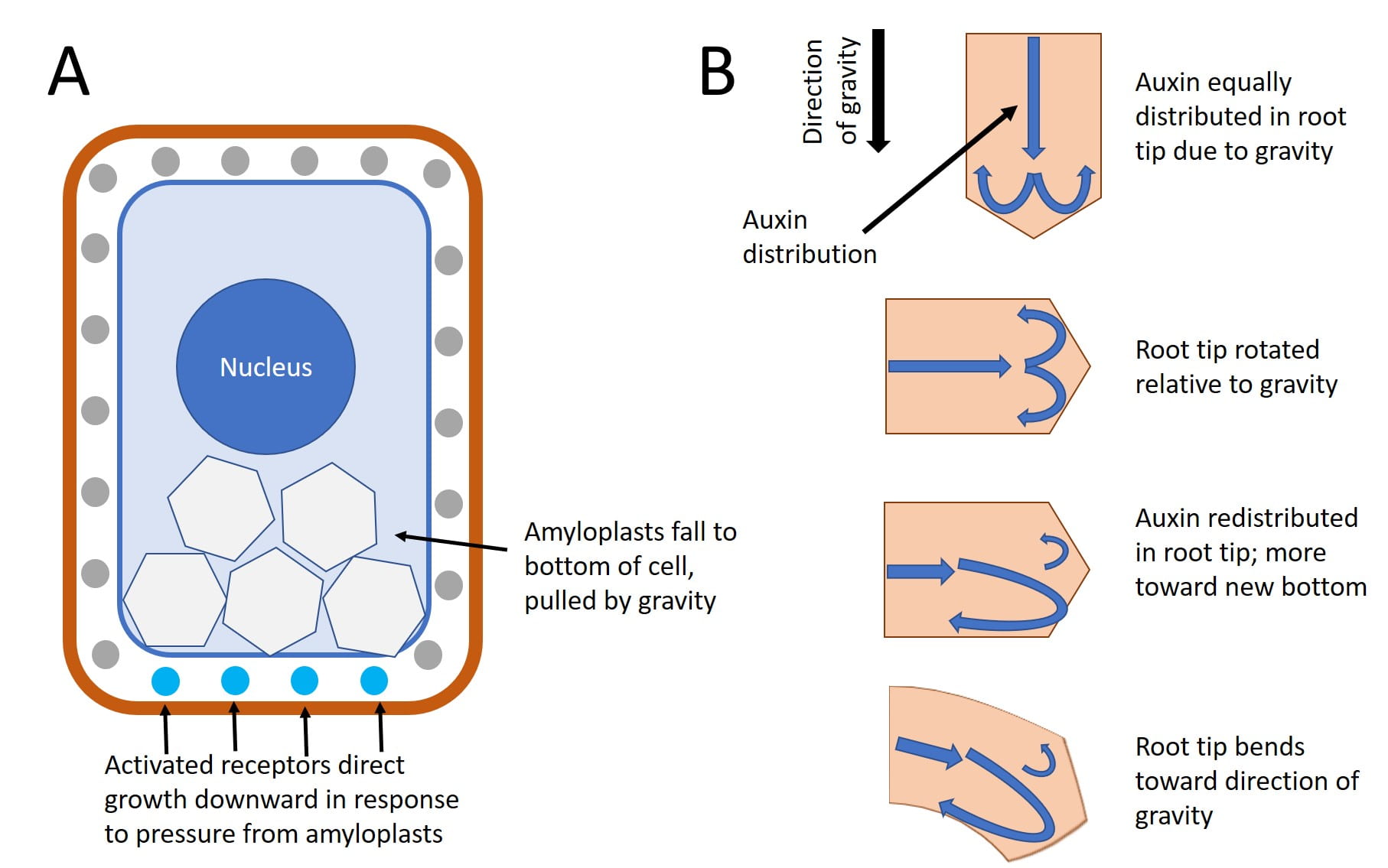
Amyloplasts (also known as statoliths) are specialized cellular compartments that contain starch granules that move in response to gravity. The starch granules are heavy, and they literally fall to the bottom of the cell in response to gravity. Amyloplasts are found in shoots and in specialized cells of the root cap. When a plant is tilted, the statoliths drop to the ‘new’ bottom cell wall, which causes auxin (produced by the root apical meristem just like at the shoot apical meristem) to redistribute to the new bottom of the root. In roots, a high concentration of auxin inhibits cell elongation, slowing growth on the lower side of the root, while cells develop normally on the upper side and causing the root to bend toward the high concentration of auxin and thus causing the root to grown down. Note that this is the exact opposite of auxin’s affect on shoots, where a higher concentration stimulates cell expansion, causing the shoot to bend away from the higher concentration of auxin. After root begins to grow vertically again, the amyloplasts return to their normal position and auxin is equally distributed on both sides of the root tip.
Growth Responses
Other plant responses to different growth-related stimuli include:
- Apical dominance: many plants grow primarily at a single apical meristem and have limited lateral branches (which would result in multiple meristems). This phenomenon is called apical dominance, and is regulated by the presence of auxin at the apical meristem. Auxin is required for the function of other growth-regulating hormones such as cytokinins; cytokinins promote cell division, but only in the presence of auxin. Abscisic acid in the lateral buds inhibits production of auxin, and removal of the apical bud will release this inhibition of auxin, allowing the lateral buds to begin growing.
- Leaf abscission: some plants drop leaves in response to changing seasons (based on temperatures, photoperiod, water, or other environmental conditions). This process is called leaf abscission, and is regulated by interactions between auxin and ethylene. During the growing season, the leaf produces high levels of auxin which blocks activity of ethylene; however, as the seasons change, the leaf produces lower levels of auxin. Lower levels of auxin permit ethylene to initiate senescence (aging) and ultimately programmed cell death at the site of leaf attachment to the stem, allowing the leaf to fall off in a controlled manner without harming the rest of the plant.
- Fruit growth: growth of fruits in size is promoted by gibberellins. Artificial addition of gibberellins to fruits while still on the plant will cause them to grow larger than they ordinarily would.
- Fruit ripening: once fruits have grown to the appropriate size, they begin ripening; this process is stimulated by ethylene. Fruit ripening is a form of senescence (aging), so the role that ethylene plays in fruit ripening is very similar to its role in leaf abscission.
Responses to Water or Water Stress (Drought)
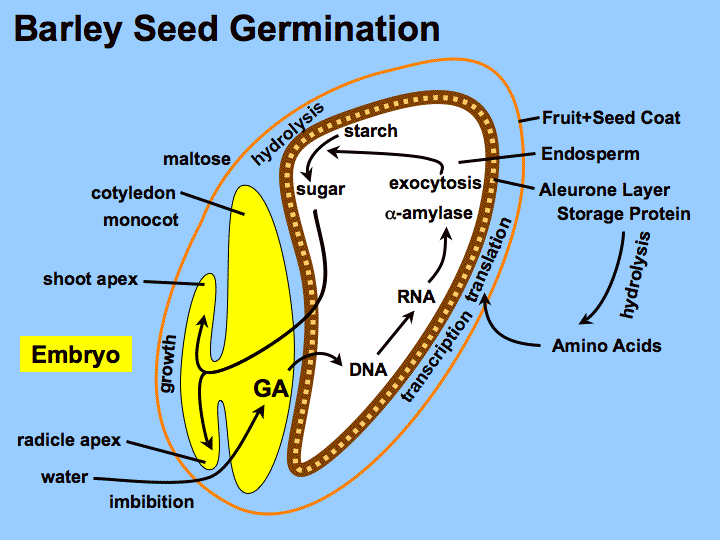
- Germination : though we previously discussed germination controlled by the phytochrome system, the seeds of some plant species instead rely on the imbibition (intake) of water to initiate germination (shown below). Intake of water activates the hormone gibberellin, which then signals to transcribe the gene encoding amylase, an enzyme that breaks down starches stored in the seed into simple sugars, and then germination proceeds (note these final steps are identical to what occurs in phytochrome-regulated germination). When water is absent, germination in this pathway is blocked by a hormone called abscisic acid (also called ABA), which inhibits the activity of gibberellins. Thus gibberellins and abscisic acid act in opposition in regulating the the germination response.
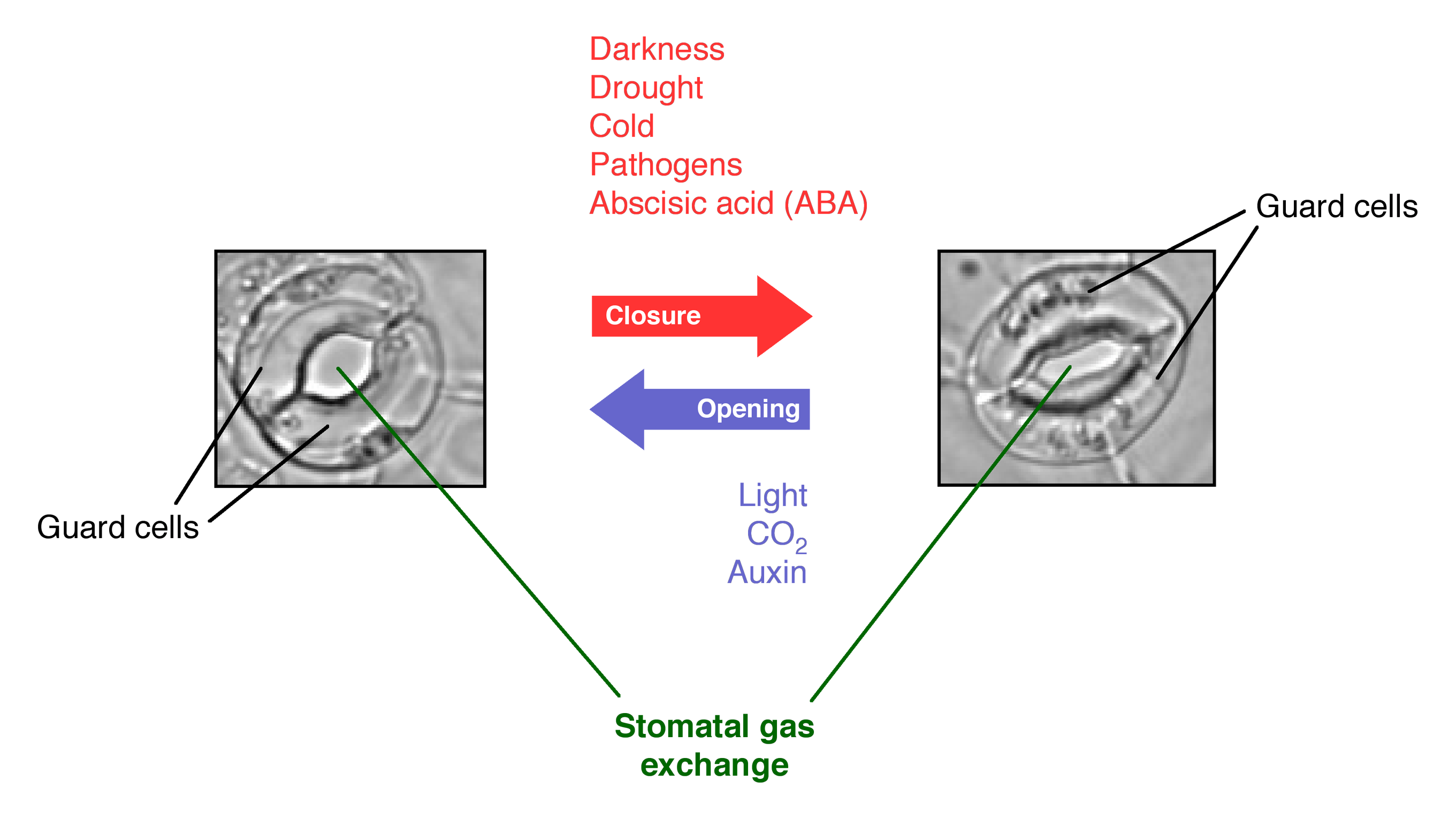
- Stomatal closing: as briefly noted above, activation of phot1 and phot2 by blue light cause stomata to open to permit gas exchange so that photosynthesis can occur. But in addition to sunlight and carbon dioxide, photosynthesis also requires water. When the plant is dehydrated due to drought, the hormone abscisic acid (ABA) causes stomata to close, preventing gas exchange and halting photosynthesis. This response to abscisic acid occurs even if blue light is present (Signaling from drought via ABA overrides the signaling from blue light via phot1).
- Local cell death: in drought conditions, the immediate response is closing stomata, as noted above. However, because closed stomata prevent gas exchange, plants will die if the stomata remain closed for too long. Thus if a drought persists for too long, the plant will begin sacrificing certain areas by allowing the leaves or stems to die in localized regions. This process may be regulated by the hormone ethylene, which can induce localized cell death under certain conditions.
Responses to Touch: Thigmotropism
Thigmotropism is movement in response to touch. Different plant species have different types of responses to touch, including slow thigmotropism and fast thigmotropism.
- Slow thigmotropism describes a plant response to a touch stimulus that affects direction of growth, such as vines that wrap around or grow along structures. Slow thigmotropism is regulated by auxin, which redistributes in the elongating stem in response to the touch, ultimately resulting in differential cell elongation (much like the role auxin plays in phototropism).
- Fast thigmotropism only occurs in a few plant species, and describes a rapid plant response to touch such the way the Venus flytrap snaps shut to trap an insect, or the way mimosa plants clamp their leaves closed in response to touch. This response occurs as a result of an electrical signal (much like in animal nervous systems!) which causes rapid changes in cell turgor pressure and thus rapid movement of structures associated with those cells.
This video shows an example of slow thigmotropism (mediated by auxin) in morning glory plants, which require a support structure of some type to grow optimally. The time lapse images were taken at 10 minute intervals:
And this video shows an example of fast thigmotropism (mediated by membrane potential) in a Venus flytrap:

